The Perthes’s disease is a rare variety of idiopathic femoral head necrosis. It is best described as an aseptic noninflammatory self-limiting idiopathic avascular necrosis of capital femoral epiphysis in a child. It is characterized by varying degrees of necrosis of the femoral ossific nucleus. It can lead to progressive permanent femoral head deformities and premature osteoarthritis. LCPD was first described by Legg, Calvé and Perthes et. al. independently in 1910, hence it is also known as Legg–Calve–Perthes disease (LCPD).
Even more than hundred years after the description of Legg- Calvé-Perthes disease, the aetiology remains to be clarified, and a great deal of confusion exists regarding the treatment of this enigmatic disease.
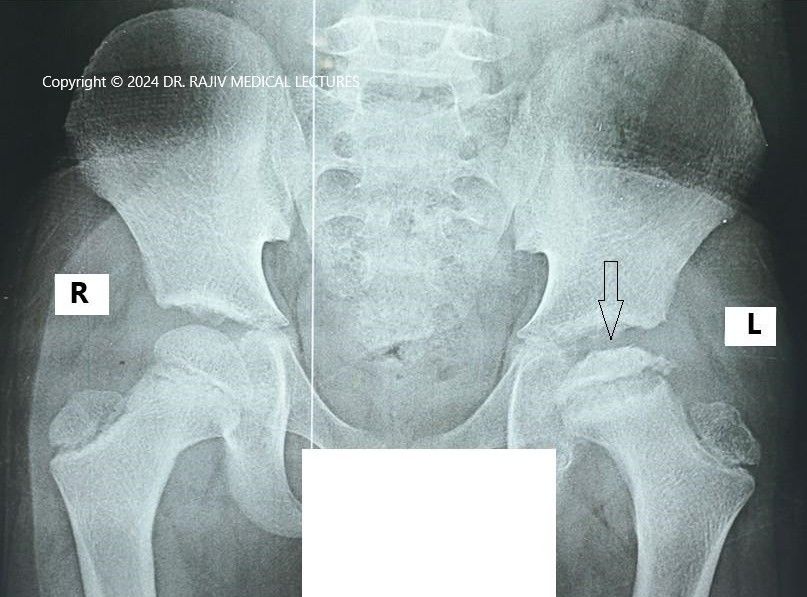
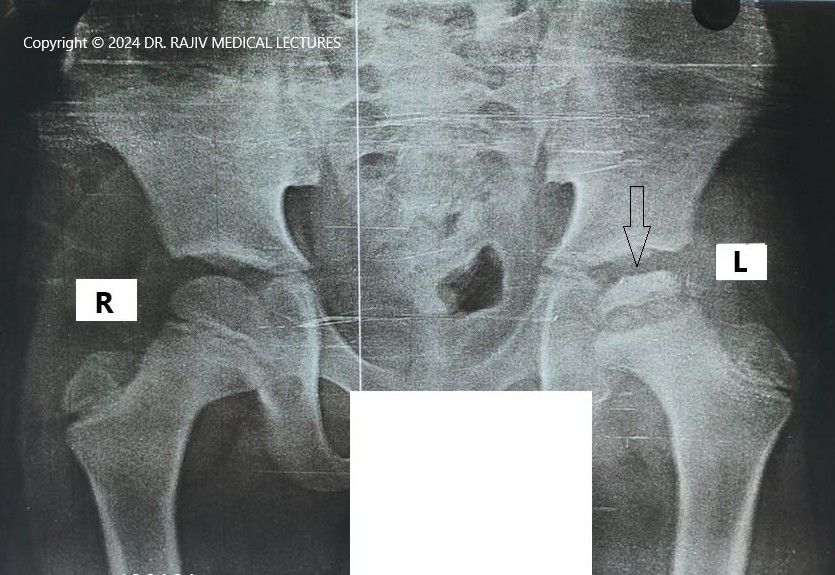
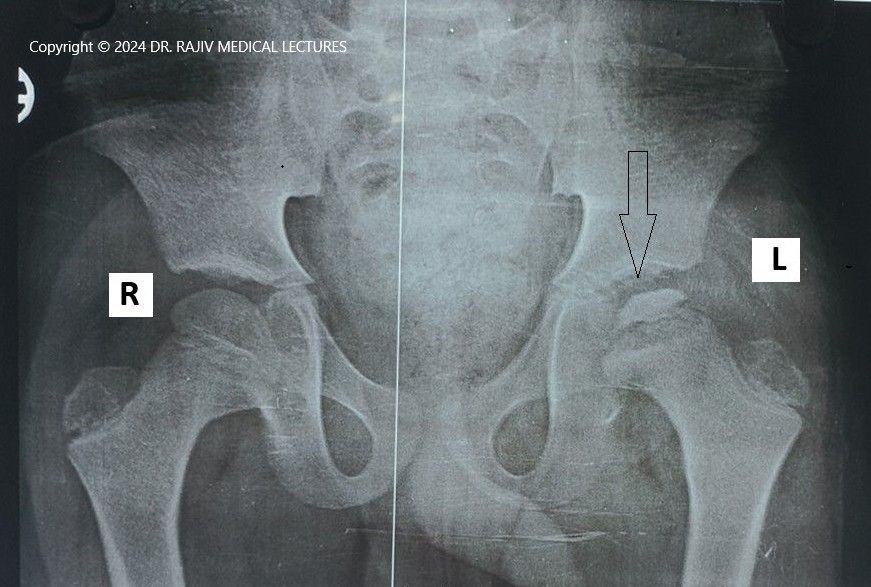
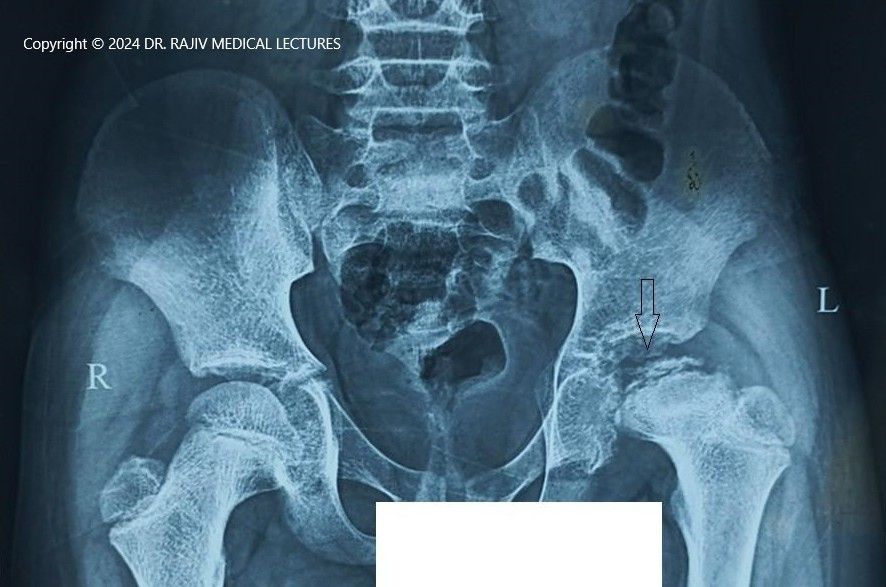
Incidence and Aetiology
Perthes disease usually affects children between the age of 2 and 15 years, predominantly males (M: F=3-5:1). The peak age of onset is around 8 years of age. Approximately 10-25% of cases are bilateral.
The cause of LCPD is unknown. LCPD has been thought to be an inflammatory disease, secondary to trauma or a developmental disorder. LCPD has a multifactorial etiology. Environmental, metabolic and genetic factors are supposed to be involved. Factors involved in the pathogenesis of Perthes disease could be low birth weight, delayed skeletal maturation, short stature with rostral sparing and last but not the least socioeconomic deprivation.
Condition may be associated with genital urinary tract abnormalities, inguinal hernia, and minor congenital abnormalities. Epidemiologic studies reveal an incidence of a positive family history of about 10%. There is a high association of breech or transverse lie, in affected patients. There are also racial and ethnic factors.
Perthes disease is found to be more common in Japanese, Eskimos, and Central Europeans.
An increased incidence of LCPD is seen in later born children, particularly the third to the sixth child, and in lower socioeconomic groups.
Pathophysiology of Perthes disease
The most widely accepted theories point to interruption of the blood supply to the femoral head. Ischemia with consequent aseptic avascular necrosis of the femoral head is a key pathogenic event in Legg-Calvé-Perthes disease (LCPD. It is a self-limiting paediatric orthopaedic condition.
Imaging, necropsy, and biopsy studies of patients with LCPD show evidence of tissue necrosis consistent with ischemic injury to the affected femoral head. According to recent studies the cause of the vascular insult may be disturbed venous drainage, intraosseous venous hypertension, or increased blood viscosity leading to decreased blood flow.
Histologic changes in the epiphyseal and physeal cartilages were described as early as 1910 by Perthes. The superficial cartilage covering the affected femoral head is thickened. The middle layer of the epiphyseal cartilage shows areas of extreme hypercellularity, while in other areas, there is a loose, fibrocartilaginous-like matrix. Areas of small secondary ossification centers are seen. The bone trabeculae are of uneven thickness forming directly on the abnormal cartilage matrix.
There is evidence of cleft formation in the physeal plate with amorphous debris and extravasation of blood.
In the metaphyseal region, there are areas where the proliferating cells are separated by a fibrillated cartilaginous matrix that does not calcify. In these areas the cells do not degenerate but continue to proliferate without enchondral ossification. This results in “tongues” of cartilage extending into the metaphysis as bone growth proceeds in other areas.
The anthropometric, epidemiologic, radiographic, and histologic evidences support the concept of a ‘susceptible child’. Perthes’s disease may, therefore, represent a localized manifestation of the generalized transient defect of the epiphyseal cartilage, that clinically manifests in the proximal femur because of its unusual and vulnerable blood supply.
Natural History and Clinical Features
Long-term studies on the natural history of Perthes disease are few. Most commonly patients present with a history of slow onset of a limp. Most of the patients are active and asymptomatic even if their femoral head is deformed.
Pain when present is usually activity related. It is relieved by rest and analgesics. Its nature is mild and, most patients do not seek medical advice until weeks or months after the onset of disease.
The pain is typically localized to the groin may refer to the anteromedial thigh or knee region. Failure to recognize this referred pain to the thigh or knee may cause further delay in the diagnosis. Some children may present with more acute symptoms.
Children with LCPD usually present with restricted hip motion, particularly abduction and medial rotation. Early on during the disease, the limited abduction is caused by muscle spasm of the adductor muscles. As the time elapses, radiological evidence of hip deformities may develop subsequently. Limitation of abduction may become permanent. Long-standing adductor spasm may lead to adductor contracture.
The Trendelenburg test is often positive. Most patients have evidence of disuse atrophy of thigh, calf, and buttock due to inactivity secondary to pain. This also helps in detecting the neglected cases. Limb length should be measured; and a discrepancy indicates significant head collapse and a poor prognosis.
The patient’s height, weight, and bone age may also help in the differential diagnosis. After the disease is established, it follows a well-described course.
All or part of the femoral epiphysis may become affected by avascular changes. The ischaemic bone may collapse, followed by revascularisation, resorption and fragmentation of the dead ossific nucleus within the cartilaginous femoral head. Finally, there is reossification and regeneration leading to ‘healing’ of the bony epiphysis. Shape change in femoral head is irreversible and there is a permanent defect in the hip joint.
In a study with an average follow-up of 36 years (range 30—48 years) Gower and Johnston demonstrated that 6 out of the 36 have had surgical procedure(s) as an adult (diagnostic biopsy, subtrochanteric osteotomy, bone grafting of the femoral head, or cup arthroplasty).
In other patients, there was typically mild limp, minimum shortening, absent or mild hip pain, and minimum or no functional impairment with respect to activities of daily living. The Perthes’s disease is self-limiting as blood supply to the affected area restores over a period. The natural history of Perthes disease consists of two phases:
(1) Evolution phase, and
(2) Healing or Remodeling phase.
Evolution Phase
In this phase there is sclerosis of the femoral head epiphysis which is small and radio dense. The epiphyseal plate is uneven, and the metaphysis blurred. There is often widening of the medial joint space due to synovitis and relative cartilage overgrowth. This progresses to fragmentation, of the bony epiphysis which appears to be in pieces.
Healing and Remodelling Phase
In next phase there is healing of the epiphysis with gradual repair of the fragmented femoral head to a homogeneous epiphysis which continues to grow and there is ossification of the femoral head. The bone quality returns to normal, however, there may be gross deformation of the femoral head and neck, and the acetabulum.
Diagnosis of Perthes disease
The initial diagnosis of Legg-Calvé-Perthes can be difficult, unless a high degree of suspicion is maintained. Symptoms may be present on an average 6 weeks before the diagnosis is made. The period between onset of symptoms and diagnosis may be longer if the pain is mild, or patient presents late, or the clinician is not wary. The diagnosis is generally made on the basis of the clinical and plain film findings.
Radiographic changes of the femoral head (such as sclerosis) generally occur 6 weeks after the initial symptom. Therefore, in suspicious cases, a follow-up radiograph should be obtained after 6 weeks if the child is still symptomatic.
After the diagnosis, the disease is followed by the anteroposterior and frog-leg lateral plain radiographs.
The surgeon can determine the stage of the disease and the extent of epiphyseal involvement from serial plain radiographs.
Based on plain radiographic appearances, the LCPD has been divided into FOUR STAGES.
Stage-I : Avascular Necrosis.
Stage-II : Fragmentation.
Stage-III: Regeneration (Reossification) (Reparative phase).
Stage-IV: Healing (Residual phase).
Stage-I is characterised by the failure of the ossific nucleus to grow when compared with the unaffected hip and widening of the medial joint space caused by hypertrophy of the articular cartilage of the femoral head. There is a relative increase in radiodensity of the femoral ossific nucleus(sclerosis) in relation to the femoral neck. A subchondral radiolucent zone (crescent sign/Caffey sign) may be present.
In the Stage-II or fragmentation phase, there may be resorption of the ossific nucleus.
In the Stage-III (Regeneration phase) radiodensity of the ossific nucleus return to normal, until the lesion is completely healed in Stage-IV.
Magnetic resonance imaging (MRI) can infarction, but it cannot accurately demonstrate the stages of the disease.
Its role in the diagnosis and management of LCPD has not yet been defined.
Laboratory studies are generally not helpful in LCPD diagnosis; however they may rule out other conditions.
Classification of Perthes Disease
The classifications for LCPD can be divided in two categories (1) one that defines the stage of the disease and (2) the other that tries to predict prognosticate outcome. Three prognostic classification systems, namely the Catterall, Salter-Thompson, and Lateral Pillar have been proposed based on the radiographic features during the stage of fragmentation.
Catterall Classification of Perthes’s Disease
Historically, the Catterall classification categorised patients with LCPD, based on the amount of femoral head involved during stage of fragmentation.
Group I – partial head or less than half head involvement, no sclerosis is seen.
Group II – Involvement of the anterior epiphysis with a central sequestrum, sclerosis present.
Group III – More than half head involvement, sequestrum formation and sclerosis.
Group IV -Involvement of the total head.
The Catterall classification additionally described radiographic “head at risk” signs that place the femoral head at particular risk and portend a poorer prognosis:
1.Lateral subluxation of the femoral head from the acetabulum (lateral head subluxation),
2.Gage sign (a V-shaped lucency or defect in the lateral epiphysis),
3.Presence of speckled calcification lateral to the capital epiphysis,
4.Metaphyseal cysts, and
5.A horizontal physis.
Treatment recommended by Catterall is containment of head by femoral varus derotational osteotomy for older children in groups II, III, and IV showing “head-at-risk” signs. But 1. any group I children, 2. an already malformed femoral head, 3. any child without the head-at-risk signs and 4. delay of treatment of more than 8 months from onset of symptoms are contraindications to this approach.
Salter and Thompson Classification of Perthes Disease
The Salter-Thompson classification advocated determining the extent of involvement based on the extent of the subchondral fracture in the superolateral portion of the femoral head ( the radiographic crescent sign).
Two groups described are:
Group A: When the crescent sign involves less than 50% of the femoral head (including Catteral groups I and II).
Group B: When the crescent sign involves more than 50% of the femoral head (including Catterall groups III and IV).
The later (Group B) has a better prognosis. Salter and Thompson recommended an innominate osteotomy in these patients.
Lateral Pillar Classification of Perthes Disease
Presently, the most used classification is Herring’s Lateral Pillar Classification. This system is based on the height of the lateral pillar which is defined as the lateral 15% to 30% of the epiphysis. Herring’s classification was originally a 3-category system: groups A, B, and C.
A recent addition of group B/C border makes this a 4-category system.
Group A: No loss of the lateral pillar height.
Group B: At least 50% of lateral pillar height intact.
Group C: < 50% of lateral pillar height maintained.
If a patient of group B progresses to group C or lies in between in a “gray” area, then he is designated as pillar group B/C border. This classification system does reflect the extent of the femoral head deformity.
The lateral pillar classification has been shown to have better interobserver reliability than the Salter-Thompson and Catterall classification systems. The classification has been shown to have good prognostic value and reproducibility.
Group A patients have been seen to have uniformly good outcomes and group C patients have the worst results.
Most group C patients have aspherical femoral heads, regardless of age at onset or type of treatment.
Pillar group B/C border have intermediate outcome.
The Main advantages of Herring’ classification are: it is useful during the active stages of the disease, and it allows accurate prediction of the natural history and treatment methods.
Bilateral Involvement
In approximately 10% of patients radiological changes are seen in both hips simultaneously. Bilateral cases have more severe involvement than patients with unilateral disease because most fall in a Catterall III or IV or a Herring B or C category. Both hips show radiological changes, though not necessarily at the same stage or severity. One hip is usually more affected than the other.
Bilateral involvement should be distinguished from multiple epiphyseal dysplasia of the hip.
Bone age should be determined for this. This is delayed in Legg-Calvé-Perthes disease but not in the later.
Differential Diagnosis
- 1. Septic Arthritis.
- 2. Tuberculosis of Hip.
- 3. Transient synovitis.
- 4. Juvenile Rheumatoid Arthritis.
- 5. Multiple Epiphyseal Dysplasia (MED).
- 6. Meyer Dysplasia.
- 7. Sickle Cell Disease.
- 8. Cretinism (Hypothyroidism).
- 9. Spondyloepiphyseal Dysplasia (SED).
- 10.Gaucher’s Disease.
Treatment of Perthes Disease
The factors that determine the treatment methods include:
- Age of the child at the onset of symptoms.
- Presence or absence of extrusion of the femoral head.
- Range of motion of the hip.
- Stage of the disease.
Treatment should be initiated well before the stage of fragmentation. Two factors widely used for treatment planning, are the state of the lateral pillar as per Herring’s grading and the extent of epiphyseal deformation as per Catterall’s grouping are two factors that are widely used for treatment planning. The purpose of treatment is to minimize femoral head deformity and thus to avoid the development of secondary acetabular dysplasia. This may be achieved by maintaining a good range of joint movement, using analgesia and physiotherapy as required.
The routine use of crutches and/or wheelchairs promote a flexion/adduction posture, hence using these should be discouraged. Recent literature suggests that bracing on its own does not alter the natural course of the disease. The role of operative treatment is debated.
Surgery may be performed early to prevent head deformation or late as a ‘salvage’ when deformity is limiting movements.
MRI is indicated before surgery to determine:
- if any flattening of the femoral head is already present and
- how much subluxation is present and
- how much surgical containment is necessary.
Head flattening is a contraindication for most osteotomies of any type.
Why to Treat Perthes Disease?
Perthes’s disease is a self-limiting disease. The interruption of the blood supply to the femoral head is temporary and complete revascularization of the epiphysis is the norm, if the onset of disease is before 12 years of age. In this group of patients, no treatment is required to enhance the process of revascularization. While the blood supply to the epiphysis is reestablished, the necrotic bone is completely replaced by healthy new bone by a process of “creeping substitution“
Treatment is needed in those few children in whom revascularization proceeds over a period of 2 to 4 years and so the femoral head may become deformed. These susceptible children need treatment to prevent the femoral head from becoming deformed.
Treatment in Early Fragmentation Stage
Femoral head deformation is the main complication of Perthes’ disease. Two important factors are related to the cause and timing of this complication.
- Extrusion of the femoral head is tha major factor leading to femoral head deformation.
- Deformation of the femoral head takes place in the latter part of the fragmentation stage.
Femoral head deformation may be prevented if extrusion is reversed or prevented in the early stage of fragmentation than if done later.
Treatment Options are:
- Preventing the femoral head from weight bearing forces across the acetabular margin by either preventing or reversing extrusion of the femoral head.
- Minimizing stress on the femoral head by avoiding bearing weight on the limb.
- Preventing the bone from becoming weak by reducing the osteoclastic resorption of the necrotic bone (still experimental).
Currently the most widely practiced option is the first one. It is based on the principle of “‘containment.” The goal is to allow remodeling of the femoral head. The two most recommended containment methods are femoral osteotomy and innominate osteotomy. Containment works best if arthrogram demonstrates a containable congruent hip joint and there is near-normal abduction under general anesthetic preoperatively. It is an intervention that aims to position the anterolateral part of the femoral epiphysis within the acetabulum. This protects the epiphysis from deforming stresses.
One method of containment is positioning the femur either in abduction and internal rotation or in abduction and flexion which can be done by casting, bracing, or surgery on the femur. Alternatively, by an osteotomy of the pelvis that reorients the acetabulum in a way that covers the anterolateral part of the femoral epiphysis (such as Salter osteotomy or triple innominate osteotomy) or by creating a bony self over the uncovered part of the epiphysis.
Age at onset is the most important determinant for deciding containment or no containment.
A word of caution is- if possible, avoid surgery for Legg-Calvé-Perthes disease in its active phase because of higher risk of complications.
Treatment under 8 years
If the age of onset is under 8 years, there is no extrusion of the femoral head, and the range of mot ion is good then ‘no containment’. Just preserve the range of motion and monitor for extrusion with radiographs every 4 months. If in this group, there is extrusion of the head is present, and the range of motion is good contain as soon as possible. In this case, if the range of motion is poor, restore motion by traction or abduction cast for 6 weeks followed by containment as soon as the motion is restored. In this age group the most common procedure is proximal femoral varus osteotomy.
Treatment at 8 years or above
In children, 8 years or older, irrespective of whether extrusion is present or not, contain as soon as possible if the range of motion is good; but if the range of motion is poor, restore motion by traction or abduction cast for 6 weeks followed by containment as soon as the motion is restored.
In this cohort a pelvic osteotomy such as acetabular shelf osteotomy, Dega- and Salter-innominate osteotomy, is often required for adequate containment.
Treatment Late in the Course of the Disease
Some children may present late in the course of the disease, that is, in late fragmentation or reossification stage, with an already Collapsed and deformed femoral head (Catterall 3 or 4, Herring B/C or C). These children have a reduced range of motion (particularly abduction) and attempting abduction results in hinging (femoroacetabular impingement or Hinge Abduction).
Hinge abduction occurs because of extrusion of the epiphyseal segment of the femoral head. Hinge abduction should be suspected when abduction and internal rotation are lost. A fixed hinge abduction is often associated with antalgic gait, short- limb gait, Trendelenburg gait, Duchenne gait, and out-toeing or in-toeing gaits.
Valgus Femoral Osteotomy
Treatment at this stage should attempt to minimize the effects of early deformation of the femoral head that has already occurred. A valgus femoral osteotomy overcomes the hinging and places a more congruent surface of the femoral head under the acetabulum. A combined pelvic osteotomy and varus femoral osteotomy may also be used as a salvage procedure for severe Legg-Calvé-Perthes disease.
Arthrodiastasis
Apart from containment some surgeons advocate distraction of the hip joint (arthrodiastasis) by an external fixator for an average of 4 months. This is done with a view to unload the hip and facilitate the restoration of epiphyseal height.
But the reported results are not sufficiently encouraging to recommend this as the primary procedure. Apart from these, good results have been reported with acetabular augmentation by shelf procedures or Chiari osteotomies.
Treatment After Complete Healing of Perthes Disease
Many patients with Perthes’s disease are left with residual femoral head deformity (sequelae), in spite of early treatment efforts. Symptoms can be a residual limp and poor range of hip motion. The standard classification of sequelae of Legg-Calvé-Perthes disease is the modified three-group Stulberg classification based on the sphericity of the femoral head. It predicts the risk for early osteoarthritis based on hip joint shape and congruity.
In recent times, there have been attempts to re-shape the deformed femoral head and the acetabulum in this stage by safe surgical dislocation of the hip.
There are extraarticular as well as intra-articular methods to treat this condition:
Extraarticular methods
1. Intertrochanteric valgus osteotomy.
2. Intertrochanteric valgus osteotomy.
3. Non-containment acetabular procedures (Shelf acetabuloplasty, Chiari procedure
Intraarticular methods
1. Osteochondroplasty of the head and neck.
2. Femoral head reduction.
3. Excision of osteochondritis dissecans.
4. Labral repair
| Indications for reconstructive surgery in Legg-Calvé-Perthes disease in the residual-stage | |
| Condition | Procedure |
| A malformed head causing femoroacetabular impingement or “hinge” abduction. | Osteochondroplasty (cheilectomy) or a varus, valgus, or femoral head osteotomy. |
| Coxa magna | Shelf augmentation |
| Large malformed femoral head with lateral subluxation | Pelvic osteotomy |
| Capital femoral physeal arrest | Trochanteric advancement or Arrest |
| Note: Because all these procedures are for an already malformed hip, a high percentage of unsatisfactory results should be expected using above procedures. | |
Treatment of Adults Who Experienced Perthes as Children
Adults who experienced Perthes as children may present with various deformities around the hip joint, including femoroacetabular impingement (FAI), or tears of labrum, cartilage and ligament . These may lead to instability and loss of movement and function.
Total hip replacement is required if secondary degenerative arthritis develops. The surgery should be modified to deal with structural changes in the proximal femur and the acetabulum that follows Perthes’ disease. Despite a higher rate of complications, noted in some series, the survival rate of the prosthesis is good.
Prognosis of Perthes disease
The long-term prognosis for children with Legg Calve Perthes (LCP) disease is good in most cases. After 18 to 24 months of treatment, most children return to daily activities without major disabilities. The long-term natural history of LCPD is not known. Despite deformity, most patients do well in early adulthood. Radiographic and clinical osteoarthritis is increased in 20-year to 40-year follow-ups. Degenerative joint disease develops in many patients by the sixth or seventh decade of life.
Clinical and radiographic features of prognostic value include:
- Deformity of the femoral head,
- Hip joint incongruence,
- Age of disease onset,
- Extent of epiphyseal involvement,
- Extent of epiphyseal collapse,
- Extent of epiphyseal extrusion,
- Metaphyseal abnormalities,
- Acetabular abnormalities,
- Presence or absence of Catterall’s “head-at-risk” signs,
- Protracted disease course,
- Acetabular and femoral head remodelling potential,
- Type of treatment, and
- Stage during which treatment is initiated.
The younger the child at the onset of Perthes disease, the better is the prognosis. Partial or anterior head involvement portends a more favorable prognosis than whole femoral head involvement. Catterall classified the extent of epiphyseal involvement into 4 groups. He opined that 90% good results are expected in untreated patients in groups 1 and 2, while 90% of the poor results were found in groups 3 and 4.
The long-term prognosis is related to the propensity for osteoarthritis of the hip in adulthood. It is more for patients with metaphyseal defects, if disease develops late in childhood (age ≥10 y), and if there is more complex involvement of the femoral head with residual deformity. Degenerative arthritis is expected to develop in most of these patients.
References
- Loder RT, Skopelja EN. The epidemiology and demographics of Legg–Calvé–Perthes disease. ISRN Orthop 2011;1–14.
- Rodríguez-Olivas AO, Hernández-Zamora E, Reyes-Maldonado E. Legg-Calvé-Perthes disease overview. Orphanet J Rare Dis. 2022;17(1):125. doi:10.1186/s13023-022-02275-z.
- Pavone V, Chisari E, Vescio A, Lizzio C, Sessa G, Testa G. Aetiology of Legg–Calvé–Perthes disease: a systematic review. World J Orthop. 2019;10(3):145-165. doi:10.5312/wjo.v10.i3.145.
- Poul J. Diagnosis of Legg-Calvé-Perthes disease. Ortop Traumatol Rehabil. 2004 Oct 30. 6 (5):604-6.
- Gower WE, Johnston RC. Legg-Perthes disease. Long-term follow-up of thirty-six patients. J Bone Joint Surg Am. 1971 Jun;53(4):759-68. PMID: 5580033.
- Huhnstock, S., Svenningsen, S., Merckoll, E., Catterall, A., Terjesen, T., & Wiig, O. (2017). Radiographic classifications in Perthes disease: Interobserver agreement and association with femoral head sphericity at 5-year follow-up. Acta Orthopaedica, 88(5), 522–529. https://doi.org/10.1080/17453674.2017.1340040
- William W. Hay. Current Pediatric Diagnosis & Treatment. (2007) ISBN: 9780071463003 –
- Yrjönen T. Long-term prognosis of Legg-Calvé-Perthes disease: a meta-analysis. J Pediatr Orthop B. 1999 Jul;8(3):169-72. doi: 10.1097/01202412-199907000-00005. PMID: 10399117.

Leave a Reply